A variety of excellent performance of carbon material is well known, but why can carbon compose the world’s hardest substance – diamond, and form a very soft material – graphite as well? Why is graphite conductive, while diamond isn’t? Why carbon fiber is high strength? Can you tell carbonization and graphitization?
Here, we are trying to popularize some basic knowledge of carbon material for everyone, and this may help you think about all sorts of carbon material problems in a more professional sense.
Let’s take a look from the atomic, crystal and micro level:
The first is atomic level. We are familiar with the idea that all matters consists of atoms, atoms are composed of atomic nucleus and extranuclear electron, extranuclear electron revolves around nucleus, just like the earth moves around the sun. Nucleus consists of protons and neutrons, and you need to know that various amounts of protons, divided into different elements. Such as, proton number of hydrogen is 1, helium is 2, and carbon element is sixth in the periodic table, it has 6 protons in nucleus, and the numbers of extranuclear electron is same with proton numbers, that means carbon atom has six extranuclear electrons. All atom weight is concentrated in the nucleus, and the electron weight is negligible – don’t belittle the extranuclear electron, it’s the key to how atom composes substance, different physical and chemical properties are derived from it.
Extranuclear electron has a regular rotation around the nucleus, it moves in orbit according to different distance from the nucleus. And the orbit of electrons outside the nucleus – two electrons in orbit that is closest to the nucleus, eight electrons in futher orbit. Similarly, the carbon atom is composed of carbon nucleus and six extranuclear electrons, and there are two electrons in the innermost carbon atom, four in the next outer layer, as shown in picture 1.
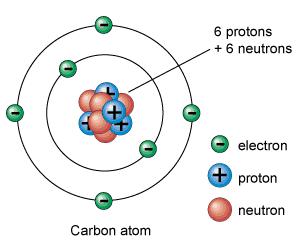
But is it mentioned there are eight electrons in futher orbit? Less then eight causes unstable, so sharing electrons with the surrounding atoms can keep stable. This means two electrons are tied together, this is called a covalent bond. Much energy is needed to break the covalent bond to separate the two electrons. A carbon atom form a covalent bond with around four carbon atoms respectively, four covalent bonds are mutually exclusive, and form the largest angle on the space, the included angle is 109° between every two covalent bonds.Whereupon, it forms the diamond atomic structure as we can see in image 2 – look the carbon atoms as balls, covalent bonds as the rigid rods that connect the balls. Any movement of a ball will lead movement the balls around, so unless all the rigid rods are broken, otherwise it is not easy to move the ball, which is reason why a diamond is so hard. In addition, the extranuclear electrons of a carbon atom form covalent bonds with other carbon atoms around outside, electrons are fixed between the two carbon nucleus, which are not free movement, even if with the applied voltage, then there is no current, and this is why the diamond is not conductive.

But why the graphite is in a hexagonal plane structure? Let’s have a look at the graphite hexagonal plane structure image (as shown in image 3) before answering this question.
It is said that a carbon atom has four extranuclear electrons, eight electrons to keep it stable, form covalent bonds with the surrounding four carbon atoms. But why the above image shows the carbon atoms only form covalent bonds with three carbon atoms? This is called the lowest energy principle – everything converts from high energy to low spontaneously, and releases the rest energy.Two adjacent carbon atoms are close in the graphite structure, so the energy is low. Carbon atoms of Graphite are in a plane, and form covalent bonds with the other three carbon atoms, the included angle of each two covalent bonds is 120 °, this structure has the lowest energy.
The structure of graphite, a carbon atom form three covalent bonds with three carbon atoms around, and the remaining electron is dissociative on the plane, each carbon atom has a redundant dissociative electrons, that is the structure of graphene. Once carbon atoms are stable, less boundage to free electrons, free electrons will directionally and rapidly move with voltage, and this is why graphene is superconducting material, with high electrical conductivity and few resistance, which is the principle of graphite material conducts. In addition, a layer of electron in carbon hexagonal plane can absorb visible light, which explains why graphite is black – light shines on the graphite and be absorbed.
The second is crystal level. What is crystal? In materials science, the atoms’ periodic permutation according to certain rules is crystal structure. Look at the structure of diamond – a carbon atom and four carbon atoms around form cube structure with periodic permutation, while the structure of graphite – carbon atoms form a- hexagonal grids with the surrounding three carbon atoms, then repeatperiodically, this is called graphite crystals. Graphite material is stacked up by hexagonal grids layer by layer, the stacking of two layers of hexagonal grids is to rely on the free electrons’ mutual attraction.This binding force is very weak, and it is easy to slide with the action of external force, which is why graphite can be used as lubricating oil additive, because two planes can slide freely, which plays a role of lubrication. And this is why a pencil needs graphite, when writing with a pencil, part of the grids plane is rubbed and paste on the paper.
Why the carbon material is corrosion resistant? Because carbon has a high liveness when combining with other elements – means it’s very difficult to react in normal temperature, so carbon material is corrosion resistant. Then why the reaction between carbon and oxygen is so intense? Why coal is easy to burn? Why the graphite oxidizes easily.
When carbon atoms combine with oxygen atoms, the original chemical combination bonds between carbon atoms should be broken, and then combine with oxygen atoms to form chemical combination bonds. Because the energy needed to break the chemical combination bonds between carbon atoms is lower than formation of carbon and oxygen atoms. That is to say, the energy released by the combination of carbon and oxygen atoms will brake the chemical combination bonds between carbon atoms, then carbon and oxygen atoms combine continuously, chemical combination bonds between carbon atoms been broken constantly – this is combustion.
Speaking of crystal structure, here comes irregular atomic arrangement – this is non crystal, like amorphous carbon. In fact, this is a relative concept, there is no perfect crystal in the world, even a single crystal has irregular atomic arrangement inside. The atomic arrangement in amorphous carbon is mixed and disorder, even with the residual unsaturated carbon atoms, that is to say, there is no stable chemical combination bonds. Then the amorphous carbon easily reacts with oxygen, because oxygen first combine with carbon atoms that without chemical combination bonds, then the combustion happens spontaneouly.
The last is micro level. As it is mentioned above that a single crystal atom is regularly arranged, if atoms of the objects are arranged regularly, that is single crystal, which is rare. Most are small single crystal particles that are made of atoms, and small single crystal particles combine into an object.
Take graphite as a example, carbon atoms form hexagonal grids, then hexagonal grids stack up as small graphite particles, graphite particle may link staggered with next graphite particle to form graphite. And covalent bonds between carbon atoms are very strong. But binding force is relatively weak in some irregular atomic arrangement or carbon atoms lacking parts, then the crack will extend along these parts.
Finally, carbon fiber, if carbon fiber is a perfect carbon hexagonal planar single crystal, then the tensile strength will reach 800Gpa! Strength of T700 carbon fiber is 4.9GPa, only about 0.5% of the theory strength. Because carbon fiber is multilayer graphite structure inside, that is to say, with graphite hexagonal grids structure, but grids plane are disorder and defective, this severely limit the strength of carbon fiber. And the hexagonal grids orientation is not completely parallel to the carbon fiber length, this also limits the strength of carbon fiber.